FAQ
What is cold corrosion and why is it so dangerous?
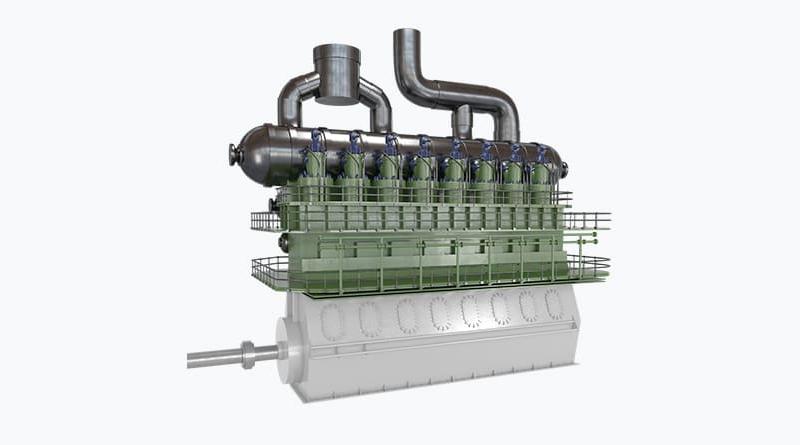
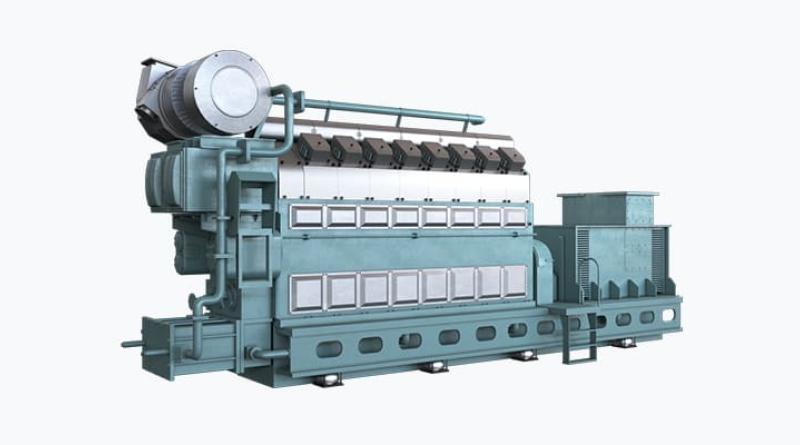
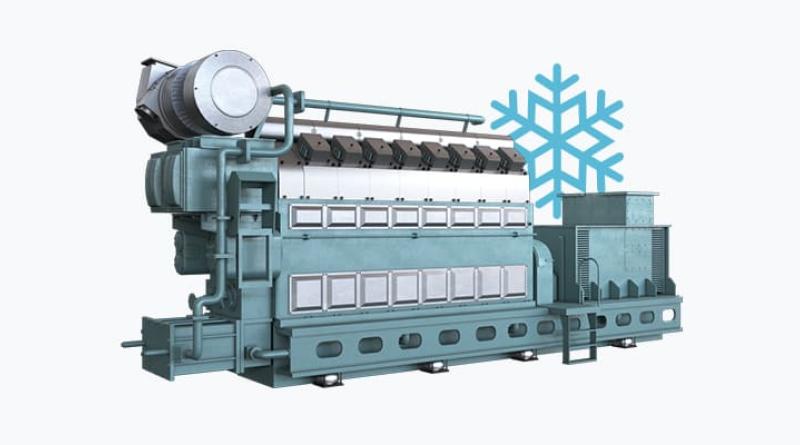
The corrosion phenomenon results from an acid attack on a liner and rings surfaces of the 2-stroke engines. The acid is produced by the combustion of sulfurized fuels - it is mainly sulfuric acid, or from the degradation of hydrocarbons of the fuel and the degraded lube oil with organic acids. It is called "cold corrosion" because the strongest events have been firstly observed on metal surfaces at low temperature, e.g. the lower part of the liner, that favors the acid condensation.
However, the corrosion can affect as well the hottest parts of the cylinder, near the combustion area. Corrosion leads to surface dissolution producing iron oxides that solubilize in the lubricant. But the most critical phenomenon is the resulting mechanical weakness and the degradation of the surface by removal of iron particles. As soon as the so-called corrosive wear happens a strong failure can be expected and liners might be lost very quickly.